Chemistry
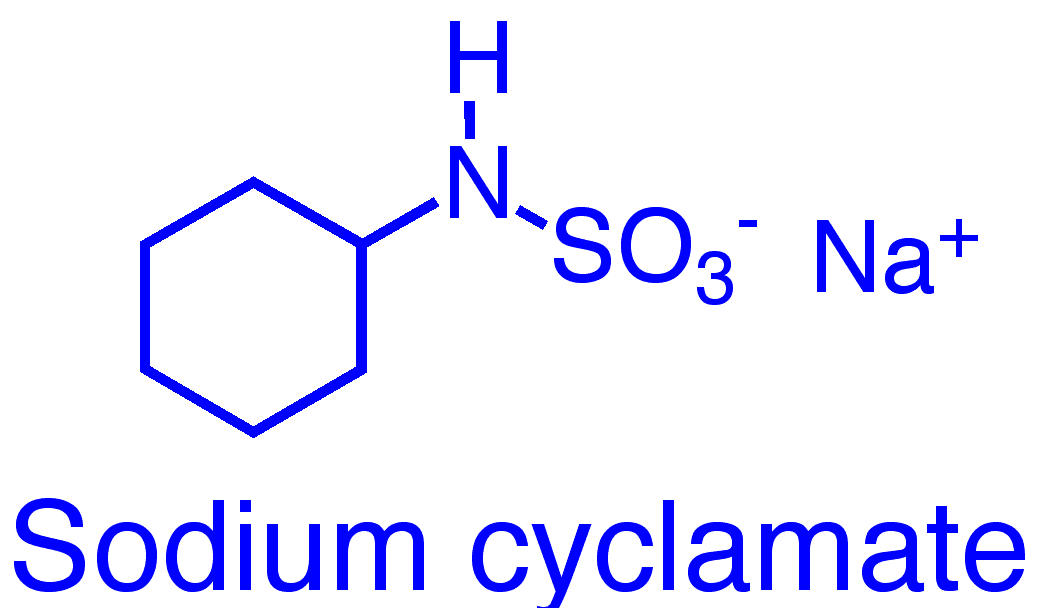
Cyclamate is a sulfamic acid, usually used as the sodium or calcium salt.
Molecular formula:
C6H13NO3S (cyclamic acid)
C6H12NNaO3S (sodium cyclamate)
C12H24CaN2O6S2 (calcium cyclamate)
Molecular weight:
179.23 (cyclamic acid)
201.22 (sodium cyclamate)
396.54 (calcium cyclamate)
Taste
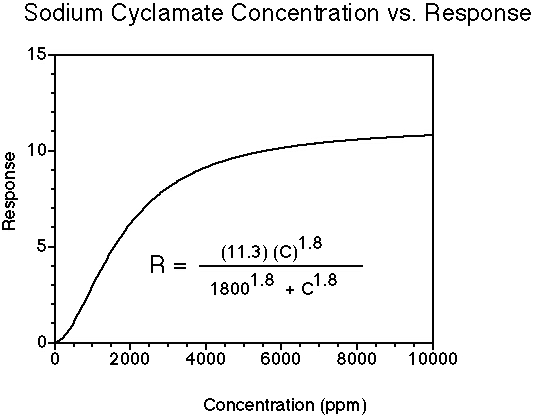
Cyclamate tastes sweet. At high concentrations, sodium and calcium cyclamate may have detectable salty taste as well. The sweetness potency relative to sucrose is about 35-50, but depends upon the concentration of sucrose which is being matched. The concentration vs. response relationship in water (results in food systems will vary) is shown below. This graph is based on data from DuBois, Walters, Schiffman, Warwick, Booth, Pecore, Gibes, Carr & Brands in "Sweeteners: Discovery, Molecular Design, and Chemoreception," D.E. Walters et al., Eds., American Chemical Society, 1991. The equation allows you to calculate sweetness response (R) for any concentration (C). The units of R are percent sucrose equivalent; the units of C are parts per million (ppm).
Safety
JECFA has established an acceptable daily intake (ADI) of 11 mg per kg of body weight for acesulfame-K. The European Food Safety Authority has set the ADI at 7 mg per kg of body weight.
Discovery
Michael Sveda was a graduate student at the University of Illinois, working in the laboratory of L.F. Audrieth on the synthesis of anti-pyretic (anti-fever) drugs. While working in the laboratory in 1937, he put his cigarette down on the lab bench. When he put it back in his mouth, he discovered the sweet taste of cyclamate.