Chemistry
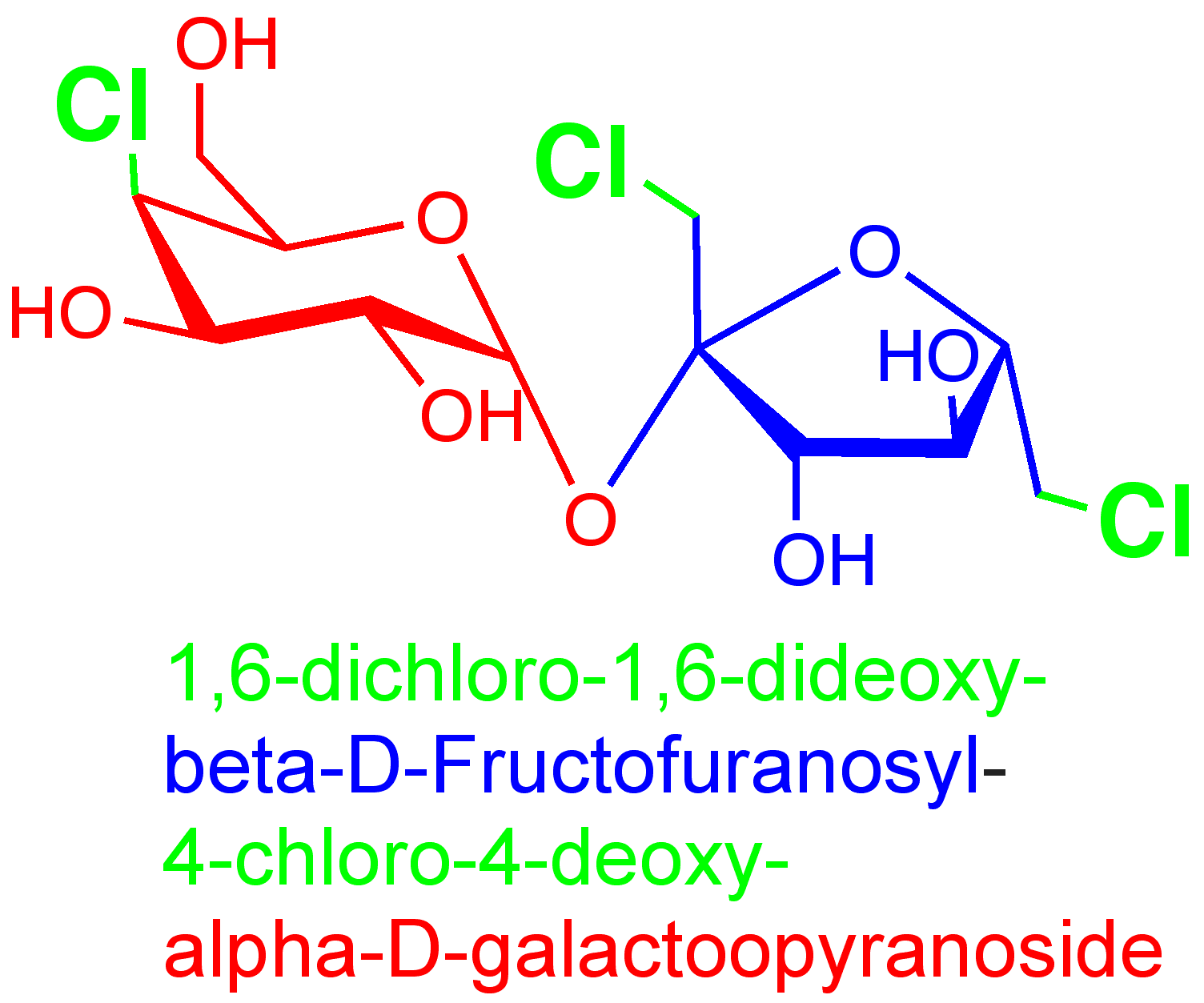
Sucralose is a sucrose molecule in which three of the -OH groups have been replaced by chlorine atoms. In the course of the chlorination, the stereochemistry at position 4 of the glucose ring gets inverted, so it becomes a derivative of galacto-sucrose.
Molecular formula: C12H19Cl3O8
Molecular weight: 397.64
Taste
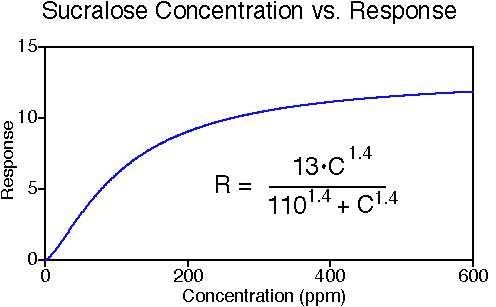
Sucralose tastes sweet, with a slight off-taste that is described as "drying" or bitter by some tasters. It has a slightly slower onset of sweetness than sucrose, and the sweetness lingers a little longer than sucrose. The sweetness potency relative to sucrose is about 600, but it depends upon the concentration of sucrose which is being matched. The concentration vs. response relationship in water (results in food systems will vary) is shown below. This graph is based on data from DuBois, Walters, Schiffman, Warwick, Booth, Pecore, Gibes, Carr & Brands in "Sweeteners: Discovery, Molecular Design, and Chemoreception," D.E. Walters et al., Eds., American Chemical Society, 1991. The equation allows you to calculate sweetness response (R) for any concentration (C). The units of R are percent sucrose equivalent; the units of C are parts per million (ppm). The sucralose data were fit to a Hill-type equation (exponential).
Discovery
Sucralose may have the strangest "accidental discovery" story of all the sweeteners. Tate & Lyle, a British sugar company, was looking for ways to use sucrose as a chemical intermediate. In collaboration with Prof. Leslie Hough's laboratory at King's College in London, halogenated sugars were being synthesized and tested. A foreign graduate student, Shashikant Phadnis, misunderstood a request for "testing" of a chlorinated sugar as a request for "tasting," leading to the discovery that many chlorinated sugars are sweet with potencies some hundreds or thousands of times as great as sucrose.