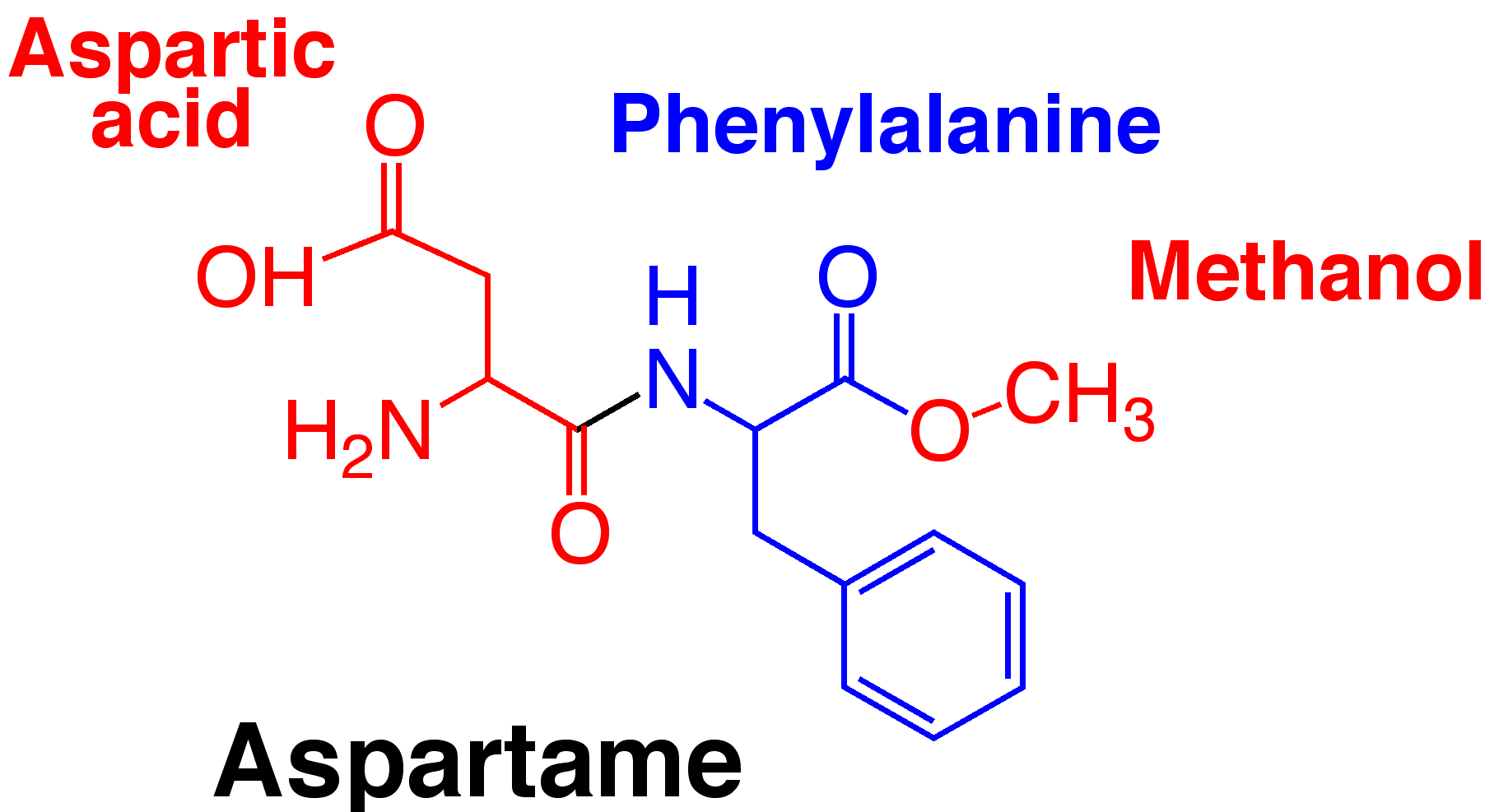
Chemistry
Molecular formula: C14H18N2O5
Molecular weight: 294.31
Aspartame is a dipeptide methyl ester.
Taste
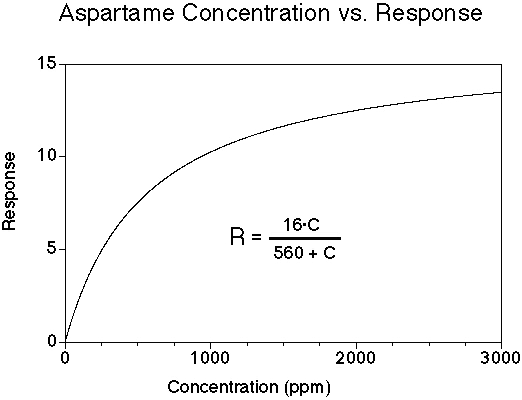
Aspartame has a sweet taste with minimal bitterness for most people. Its onset of sweetness may be slightly slower than sucrose, and the sweetness may linger. The sweetness potency relative to sucrose is about 180, but depends upon the concentration of sucrose which is being matched. The concentration vs. response relationship in water is shown below (results in food systems will vary). This graph is based on data from DuBois, Walters, Schiffman, Warwick, Booth, Pecore, Gibes, Carr & Brands in "Sweeteners: Discovery, Molecular Design, and Chemoreception," D.E. Walters et al., Eds., American Chemical Society, 1991. The equation allows you to calculate sweetness response (R) for any concentration (C). The units of R are percent sucrose equivalent; the units of C are parts per million (ppm).
Safety
I have written an essay on the metabolism of aspartame.
Discovery
It was December, 1965. Jim Schlatter, a chemist at G.D. Searle, was working on a project to discover new treatments for gastric ulcers. To test new anti-ulcer drugs, the biologists used a tetrapeptide (four amino acids) normally produced in the stomach; Schlatter was synthesizing this tetrapeptide in the lab, and one of the steps in the process was to make a dipeptide intermediate, aspartyl-phenylalanine methyl ester.
In the course of his work, Schlatter accidentally got a small amount of the compound on his hands without noticing it. Later that morning, he licked his finger as he reached for a piece of paper, and noticed a sweet taste. His curiosity drove him to ask "Where did that sweet taste come from?" His first thought was of the doughnut he had eaten during his coffee break, but he realized that he had been to the bathroom and had washed his hands since then. It could only be the aspartyl-phenylalanine methyl ester he had worked with. He knew that aspartic acid and phenylalanine, which make up this product, are natural amino acids present in all proteins, so he felt it would be safe to taste the material. It was sweet! He and his lab partner, Harman Lowrie, both tasted the material in 10 milliliters of black coffee, noting the sweet taste as well as the absence of any bitter aftertaste, and recorded the results in Schlatter's laboratory notebook. His boss, Bob Mazur, convinced the company of the potential value of this discovery. Twenty years later, Schlatter's curiosity had produced a billion dollar per year sweetener.