Is Stevia Natural?
by D. Eric Walters, Ph.D.
Is stevia natural? Someone recently asked me that question, because of a web posting that said commercial stevia sweeteners have been "extracted and processed" by big corporations, to the point that they are no longer "natural."
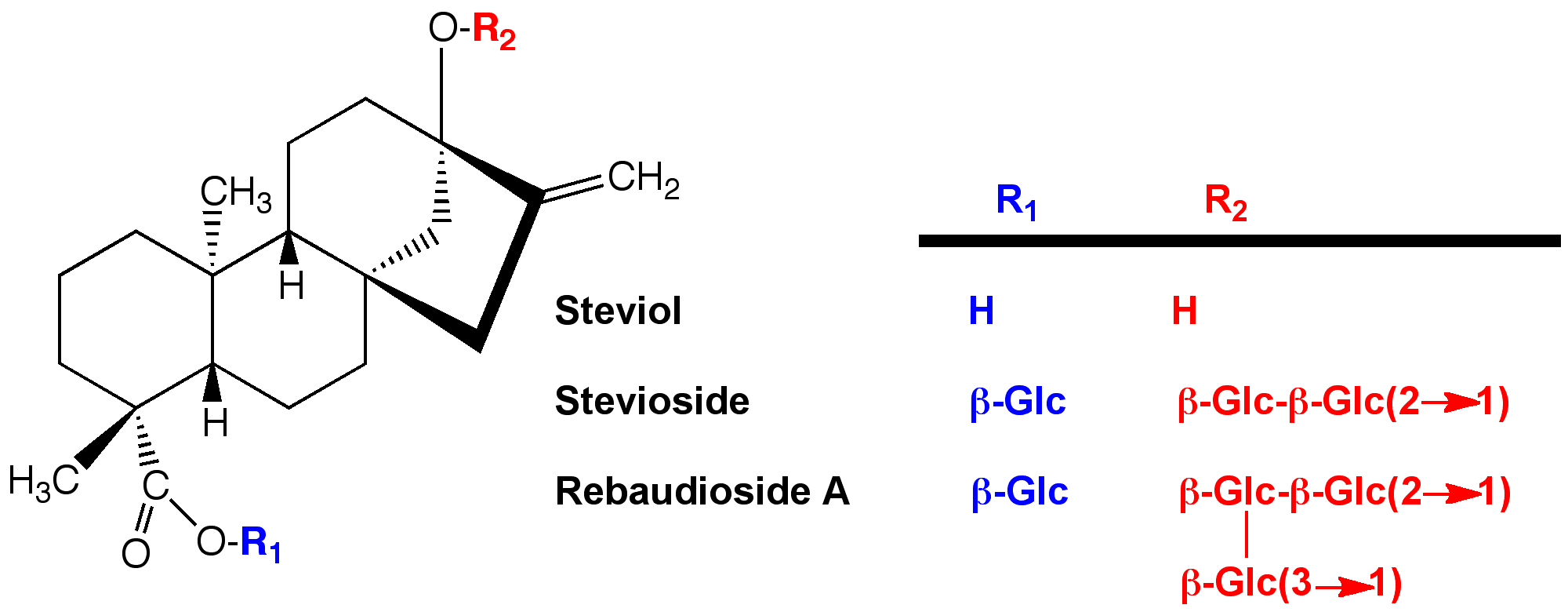
The word “stevia” is tossed around pretty loosely these days. It can refer to the plant (Stevia rebaudiana Bertoni), or to material extracted from the leaves of this plant, which may contain varying amounts of the various sweet diterpenoid glycosides that contribute sweetness. Diterpenes are plant metabolites composed of 20 carbon atoms. Plants make extensive use of the 5 carbon “isoprene” building block to make terpenes (10 carbons), sesquiterpenes (15 carbons), diterpenes (20 carbons), and even larger structures. Plants use these compounds to attract pollinators, repel parasites and predators, and for numerous other purposes.
The sweet components of the stevia leaf are composed of the diterpenoid steviol linked to one or more sugars. In the wild, the major steviol derivative is stevioside, which has 3 sugars attached. It is pretty sweet, but it also has some bitterness. Next most abundant is rebaudioside A, which has 4 sugars attached. It is a little less sweet than stevioside, but significantly less bitter. Considerable effort has been devoted to (1) increasing the amount of rebaudioside A in the plant, and (2) purifying rebaudioside A; Rebiana™ is an example. There are lesser amounts of other rebaudiosides (C, D, E, F, M) and related compounds steviolbioside (stevioside with one of its sugars removed), and rubusoside (stevioside with a different sugar removed). There are now efforts to isolate stevia glycosides that exist at even lower levels in the plant (rebaudiosides T, U, and IX have been reported). The goal is to find the ones with the best sweetness and the least bitterness. Then plant breeding is carried out to find varieties of the plant that produce greater quantities of the more desirable glycosides. Interestingly, people have steered away from the GMO approach. I think they realize that the appeal of stevia is the “natural” aspect—the taste quality is not the main attraction. In addition to the bitterness, stevia sweeteners generally have slow onset of sweetness, and a tendency for the sweetness to linger. In a practical sense, if you try to sweeten a cola using only stevia, the sour and bitter taste components arrive before the sweetness, and that’s not what consumers expect when drinking a cola.
While people are not necessarily trying to genetically engineer the stevia plant, there is a lot of work going on to use enzymes produced by yeast or bacteria to attach more sugars to the readily available stevioside, to try to improve its taste. If you take stevioside that you isolated from stevia leaves, and you use a “natural” bacterial or yeast enzyme to add “natural” sugar molecules to produce rebaudioside A, a molecule which occurs naturally, is that “natural”?
Clearly, “natural” is a word that is even more abused than “stevia.” First, just because something is natural, you cannot assume it is safe. Foxglove and belladonna are natural plants that produce highly toxic materials. Is it “natural” to extract and purify stevia glycosides from the stevia plant? I think this is more a philosophical question than a scientific one. Where do you draw the line? Would you rather “process” a walnut by taking off its shell, or eat it in its natural state? Plant breeding has been going on for millennia, with both positive and negative outcomes. Genetically manipulating yeast or bacteria to convert stevioside to rebaudioside A is a little further along the spectrum (further from "natural"), and I understand some of the concerns there. But in the end, when you have a bottle of pure rebaudioside A, it’s the same chemical structure whether it was produced solely by a Paraguayan plant or with the help of an engineered yeast. If there is a toxic effect, it will be the same toxic effect in either case. If there is a beneficial effect, it will be the same in either case. As a chemist, I am more concerned with the effects of molecules on a molecule-by-molecule basis than with where the molecule came from. The wonderful smell and flavor of baked bread arises, in part, from chemical reactions (Maillard and Amadori reactions) that take place between sugars and proteins under the influence of heat. Is that “natural”? If you were to eat raw coffee beans, you would not recognize them as coffee. They must be fermented, dried, and roasted to produce the flavor you expect from coffee. The roasting pyrolyzes fats, proteins and sugars, producing dozens of pyrazines and other aromatic chemical substances that many of us have grown to love. I suppose I could never be “Mr. Natural” if it means I have to consume raw coffee beans.